Achieve lasting change
in hand and foot atopic dermatitis*1-3
Living with atopic dermatitis on your most essential body areas like the hands and feet can make daily activities including walking and writing incredibly burdensome even in the case where disease symptoms are mild elsewhere. Treating atopic dermatitis on the hands and feet has historically been difficult to treat.

Having Dupixent data added for this difficult-to-treat population is important for physicians looking for tools to treat these patients and reinforces the already well-established efficacy and safety of Dupixent®(dupilumab) in atopic dermatitis overall.1,2
Dupixent®(dupilumab) in patients with atopic hand and foot dermatitis1-2
The Phase 3 double-blind, placebo-controlled trial, LIBERTY-AD-HAFT, evaluated the efficacy and safety of Dupixent in 133 adult and adolescent (aged 12 to 17 years) patients with atopic dermatitis with moderate-to-severe hand and/or foot involvement who had an inadequate response or intolerance to topical corticosteroids. Patients with hand and foot disease predominantly driven by allergic or irritant contact dermatitis were excluded from the trial.
The primary endpoint evaluated the proportion of patients with clear or almost clear skin of hand and feet eczema at 16 weeks, as measured by a score of 0 or 1 on the Investigator Global Assessment Scale. The key secondary endpoint measured the proportion of patients with improvement in itch on hands and feet from baseline (measured by a ≥4-point reduction in Peak-Pruritis Numeric Rating Scale [PP-NRS] on a 0-10 scale) at 16 weeks.
The Phase 3 double-blind, placebo-controlled trial, LIBERTY-AD-HAFT, evaluated the efficacy and safety of Dupixent in 133 adult and adolescent (aged 12 to 17 years) patients with atopic dermatitis with moderate-to-severe hand and/or foot involvement who had an inadequate response or intolerance to topical corticosteroids. Patients with hand and foot disease predominantly driven by allergic or irritant contact dermatitis were excluded from the trial.
The primary endpoint evaluated the proportion of patients with clear or almost clear skin of hand and feet eczema at 16 weeks, as measured by a score of 0 or 1 on the Investigator Global Assessment Scale. The key secondary endpoint measured the proportion of patients with improvement in itch on hands and feet from baseline (measured by a ≥4-point reduction in Peak-Pruritis Numeric Rating Scale [PP-NRS] on a 0-10 scale) at 16 weeks.
Figure 4. LS mean change in secondary PRO endpoints at Week 162
Baseline: Hand and Foot Skin Peak Pain NRS, mean (SD)a placebo 6.0 (n=66) dupilumab 6.8 (n=67). Sleep quality NRS (0–10), mean (SD)a placebo 5.4 (n=67) upilumab 5.5 (n=67)
Values after first rescue treatment used were set to missing. Patients with missing values at week 16 due to rescue treatment, AE, and lack of efficacy were imputed by worstobservation-carried-forward (WOCF) or baseline value if there is not any postbaseline value. Patients with missing values due to other reasons including COVID-19 were imputed by multiple imputation (MI). All non-missing data before imputation of WOCF were used for MI.
Figure 5. Secondary efficacy endpoints at Week 162
Patient was considered as non-responder after rescue treatment use or missing at each visit.
Baseline: mTLSS for hand and foot (0–36), mean (SD) placebo 16.0 (n=66) dupilumab 17.0 (n=67). HECSI (0–360), mean (SD) placebo 47.4 (n=66) dupilumab 46.2 n=67). Hand and foot PP-NRS (0–10), mean (SD)a, placebo 6.9 (n=66) dupilumab 7.2 (n=67)
NRS, Numerical Rating Scale (weekly mean of daily measure). SE, standard error; PRO, patient reported outcomes; QoLHEQ, quality of life in hand eczema questionnaire (patients with hand dermatitis only).
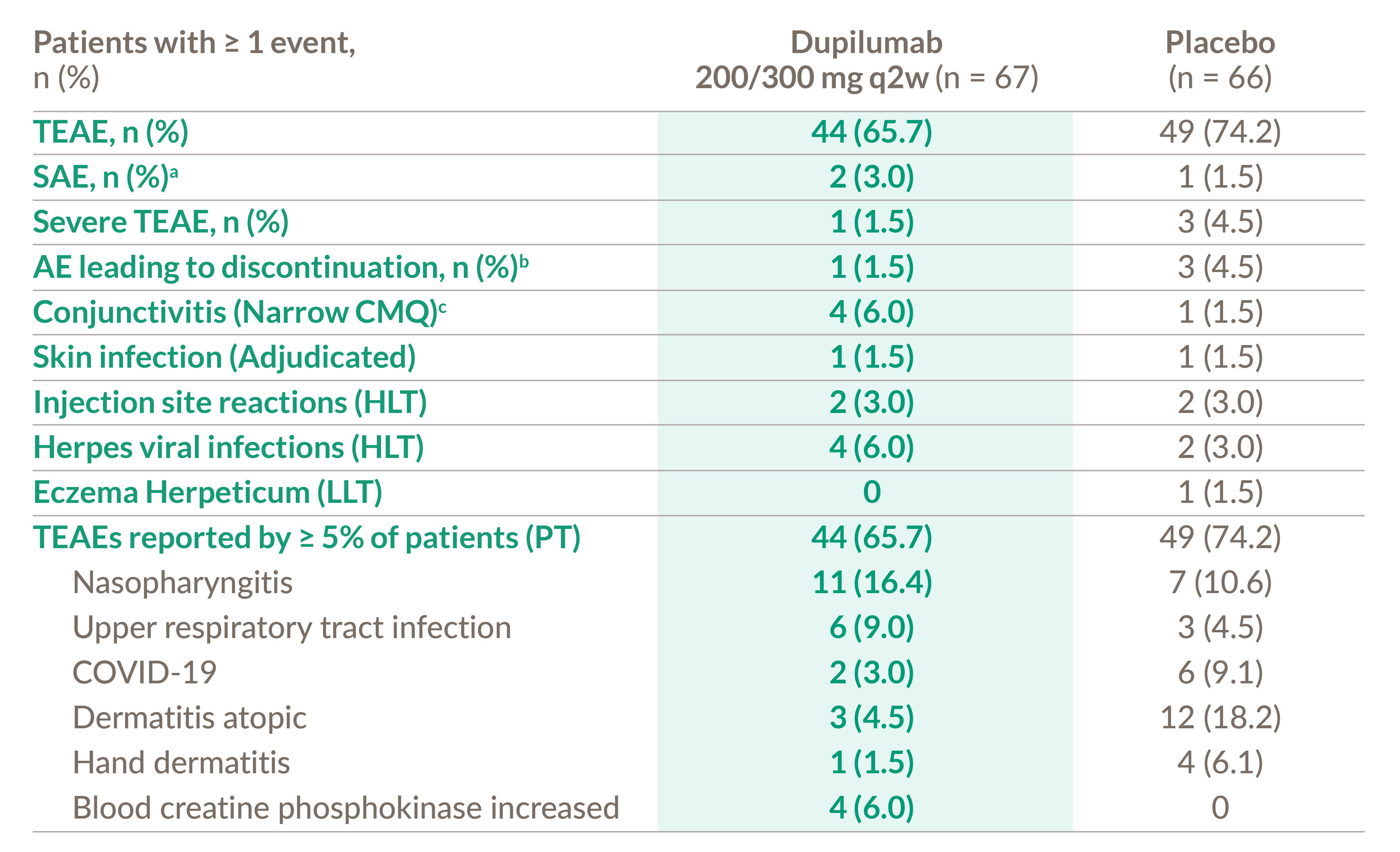
Dupixent safety profile was acceptable and was consistent with the known safety profile in the approved ad indication1,2
a) 1 patient reported appendicitis in the placebo group and 2 patients reported 3 events of post procedural infection, dizziness, and syncope in the dupilumab group.
b) 3 patients reported AEs of general physical health deterioration, COVID-19, and dermatitis atopic leading to permanent study drug discontinuation in the placebo
group and 1 patient reported AE of dizziness and syncope that lead to permanent study drug discontinuation.
c) Narrow CMQ includes PTs of Conjunctivitis, Conjunctivitis allergic, Conjunctivitis bacterial, Conjunctivitis viral, Atopic keratoconjunctivitis.
*DUPIXENT has demonstrated a significant improvement in effect and a long-term safety profile was observed up to 52 weeks in audlt patients with moderat to severe atopic dermatitis across different anatomical regions (post‐hoc analysis).3 DUPIXENT has also demonstrated a significant improvement in effect and a long-term safety profile observed up to 5 years in adult patients with moderate to severe AD.1 Sustained significant improvement in effect and a long-term safety profile was observed up to 52 weeks in patients with severe AD from 6 years to 17 years.1 Sustained significant improvement in effect and a safety profile was observed up to 16 weeks in patients with severe AD from 6 month to 5 years.1
Tables and graphs adapted by Sanofi.
**Weekly mean of daily measure.
HESCI, Hand Eczema Severity Index; mTLSS, modified Total Lesion Sign Score; PP-NRS, Peak Pruritus Numerical Rating Scale; SD, standard deviation; QoLHEQ, quality of life in Hand Eczema Questionnaire.
NRS, Numerical Rating Scale (weekly mean of daily measure). PRO, patient reported outcomes; QoLHEQ, quality of life in hand eczema questionnaire (patients with hand dermatitis only).
HESCI, Hand Eczema Severity Index (patients with hand dermatitis only); mTLSS, modified Total Lesion Sign Score; PP-NRS, Peak Pruritus Numerical Rating Scale (weekly mean of daily measure). SE, standard error; PRO, patient reported outcomes; SD, standard deviation.
CMQ, customized MeDRA queries; HLT, high level term; LLT, lowest level term; PT, preferred term;
SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Ref: 1. Dupixent SmPC, fass.se. Ref: 2. Eric L. Simpson, et al. Dupilumab Treatment in Patients With Atopic Hand and Foot Dermatitis: Results From a Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial, J AM ACAD DERMATOL, Published online February 23, 2024. Ref: 3 Blauvelt A et al. Br J Dermatol 2019;181(1):196-197
Vill du läsa mer om behandling med Dupixent för de olika indikationerna, klicka in på Fass.se
Relaterad information
MAT-SE-2400276(v1.0)april 2024